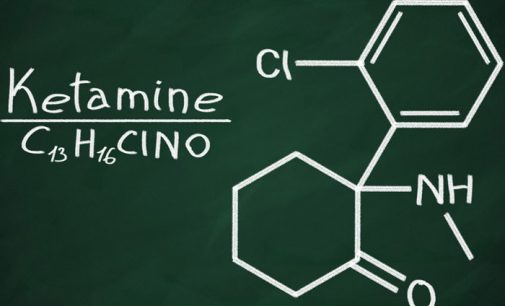
Ketamine, a medication commonly used for pain relief and increasingly used for depression, may help alleviate migraine pain in patients who have not been helped by other treatments. The study
Tag "treatment"

Veltassa (patiromer), developed by Relypsa, has received a recommendation for marketing approval from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) for the
EU regulators have expanded the scope of Janssen’s Darzalex to include patients with multiple myeloma who have received at least one prior therapy, when given in combination with lenalidomide and
Allergan, a global biopharmaceutical company, announced that it has entered into a clinical trial agreement with Novartis to conduct a Phase 2b study, using Allergan’s cenicriviroc (CVC) and Novartis’ lead FXR
BTG, a global specialist healthcare company, has received Class III CE Mark certification for DC Bead LUMI, the first commercially available radiopaque drug-eluting bead (DEB) in the EU which can
Allergan plc, a global pharmaceutical company announced topline data from a phase II study with major depressive disorder (MDD). The study evaluated the efficacy, safety and tolerability of a single administration
Scientists from LSTM’s Research Centre for Drugs and Diagnostics (RCDD) have described in a paper published today in Scientific Reports, a new way of screening potential treatments for Tuberculosis (TB)
Xeljanz (tofacitinib citrate) receives marketing authorization in the European Union for the treatment of moderate to severe active rheumatoid arthritis (RA). Pfizer Inc. announced today that the European Commission (EC)
Apeiron Biologics has announced that the EMA’s CHMP has recommended the approval of APN311 for immunotherapy of high risk neuroblastoma. Apeiron Biologics is a Vienna-based biotech company developing immunological approaches
Shire has secured label expansion approval from the European Commission for its hereditary angioedema therapy Cinryze. The European Commission (EC) has approved the label extension application, granting three new indications