The UK’s referendum decision to leave the EU in May 2016 undoubtedly sent shockwaves throughout Europe. European countries are still struggling to interpret what ‘Brexit’ will mean for them. Ireland,
Tag "EMA"

University of Liverpool researchers, working with F2G Limited (Eccles, Manchester), have developed a new antifungal drug to help in the treatment of life threatening invasive fungal infections such as invasive aspergillosis.

A retrospective cohort study, published in the British Medical Journal, has found no conclusive evidence that most oncology treatments approved for use by the European Medicines Agency (EMA) offered survival
Losing valuable staff is the major concern in the impending relocation of the European Medicines Agency. The agency which is responsible for the evaluation, authorisation and pharmacovigilance of all medicines

The Brexit-inspired need to move the London HQ of the European Medicines Agency will throw up two major risks when it comes to patients and the approval of new medicines,
The European Medicines Agency’s Pharmacovigilance Risk Assessment Committee (PRAC) discussed the safety reviews of a variety of medications during its June 2017 meeting. The medicines in review included treatments for

European regulators have accepted for review Sandoz’ marketing applications for biosimilars to AbbVie’s Humira (adalimumab) and Janssen’s Remicade (infliximab). The company is seeking approval for its biosimilars for use in

The European Medicines Agency (EMA) has approved Aptar Pharma’s integrated electronic nasal lockout device (e-Lockout) following a multi-year development with Takeda Pharmaceuticals International. Aptar Pharma agreed to supply Takeda with its
The European Medicines Agency (EMA) has approved Zebinix (eslicarbazepine acetate) for use as once-daily monotherapy to treat adults with newly-diagnosed partial-onset epilepsy. It is already indicated in Europe as an
The European Medicines Agency (EMA) met with a delegation from the East African Community (EAC) from May 18–19, 2017 as part of the agency’s collaboration with African regulators. The meeting

On May 22, 2017, the European Medicines Agency (EMA) announced that its Pharmacovigilance Risk Assessment Committee has completed an audit and evaluation of the new EudraVigilance information system for tracking

Even before European officials present the groundwork for relocating the bloc’s drug regulator, a fight has emerged between countries vying for the economic lift and status that come with hosting the

Hansa Medical has accessed the EMA’s priority medicines scheme to accelerate the development of a therapy that broadens the access to kidney transplants. Hansa Medical, based in Lund, Sweden, develops

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval to expand the use of Novartis’ Zykadia (ceritinib) in anaplastic lymphoma kinase (ALK)-positive non-small cell

Veltassa (patiromer), developed by Relypsa, has received a recommendation for marketing approval from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) for the
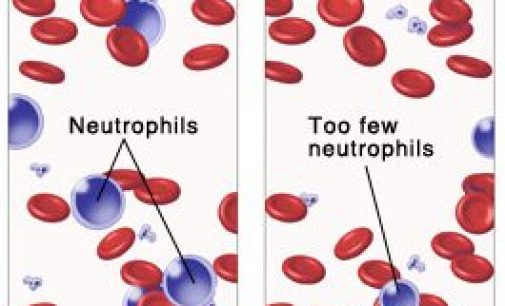
Cinfa Biotech announced trial results for a biosimilar of Amgen’s Neulasta, a multi-billion blockbuster to treat chemotherapy-induced neutropenia. Cinfa Biotech is a Spanish company specialized in developing biosimilars. The biotech

The European Medicines Agency’s (EMA) 2016 annual report focuses on the Agency’s key achievements in the areas of medicine evaluation, support to research and development of new and innovative treatments and

Ireland has stepped up its bid to host the European Medicines Agency (EMA) with the official announcement being delivered and reiterated by Minister of Health, Simon Harris over the past
EMA and the European Commission released a biosimilars information guide for health professionals during the EC’s biosimilars conference. In an effort to provide healthcare professionals with information on the science
On May 2, 2017, the EMA and European Commission published a notice alerting Marketing Authorization Holders of centrally authorized medicines of their obligations in relation to Brexit. Although the final
Contrary to some political claims, the U.S. Food and Drug Administration approved more drugs, and two to three months faster on average, than
The European Commission has approved Novartis’ Tafinlar (dabrafenib) in combination with Mekinist (trametinib) for the treatment of patients with BRAF
The European Medicines Agency (EMA) wants to suspend around 300 marketed generic meds (and applications) coming out of Indian CRO
Apeiron Biologics has announced that the EMA’s CHMP has recommended the approval of APN311 for immunotherapy of high risk neuroblastoma.
The agency is recommending the suspension of a variety of medications because of unreliable bioequivalence studies conducted by Micro Therapeutic
The move by Great Britain to step away from the European Union may be sending chills of uncertainty to many
Representatives from the European Medicines Agency (EMA) met with medicines regulators from Africa last week to discuss how to improve
Abeona Therapeutics Inc., a clinical-stage biopharmaceutical company focused on developing gene therapies for life-threatening rare diseases, announced today that the
Regulators on both sides of the Atlantic are reviewing three new submissions for medicines containing ertugliflozin, an investigational SGLT2 inhibitor
In a landmark decision, the United States FDA and the European Commission announced on March 2, 2017 that they will recognize
The European Medicines Agency (EMA) announced on Feb. 24, 2017 that its Committee for Medicinal Products for Human Use (CHMP) has
Johnson & Johnson is counting on new buy Actelion’s pulmonary arterial hypertension (PAH) meds to provide a top-line boost once








