The Centre for Process Innovation (CPI), the UK’s technology innovation provider for process manufacturing, announced it has begun the first phase of its ‘Medicines Smart Packaging’ project. This phase of
Pharmaceutical Manufacturing

The industrial robotics market is expected to be worth $71.7 billion by 2023, according to new research. The rapid growth is affecting markets across the board, from automotive and electronics

Tjoapack, a Dutch contract packaging organisation (CPO), has added an additional bottling line to its facility in Etten-Leur, the Netherlands, to meet growing demand for this service, particularly from generics

The Food and Drug Administration (FDA) has approved BioMarin Pharmaceutical’s bulk biologics manufacturing plant, located in Shanbally, Cork, Ireland for production of the formulated bulk substance (N-acetylgalactosamine 6-sulfatase (GALNS) used
Cambrex, manufacturer of small molecule active pharmaceutical ingredients (APIs), has announced it is investing in new capacity and continuous flow technology at its Karlskoga facility in Sweden. The investment includes

The European Medicines Agency (EMA) has approved Aptar Pharma’s integrated electronic nasal lockout device (e-Lockout) following a multi-year development with Takeda Pharmaceuticals International. Aptar Pharma agreed to supply Takeda with its
The European Commission (EC) has granted marketing authorisation for AstraZeneca’s Brilique (ticagrelor) orodispersible tablets (ODT) as a new method of treatment administration. This decision means that ticagrelor will become the

Researchers at Karolinska Institutet in Sweden have managed to synthesise lung surfactant, a drug used in the care of preterm babies, by mimicking the production of spider silk. Animal studies

The EC granted full marketing approval for Biogen’s Fampyra® (prolonged-release fampridine) as a treatment to improve walking in people with multiple sclerosis (MS). Clearance in Europe was based on the

On May 22, 2017, the European Medicines Agency (EMA) announced that its Pharmacovigilance Risk Assessment Committee has completed an audit and evaluation of the new EudraVigilance information system for tracking
Recordati announced that it has entered into an agreement with AstraZeneca for the acquisition of the rights to Seloken®/Seloken® ZOK (metoprolol succinate) and associated Logimax® fixed dose combination (metoprolol succinate
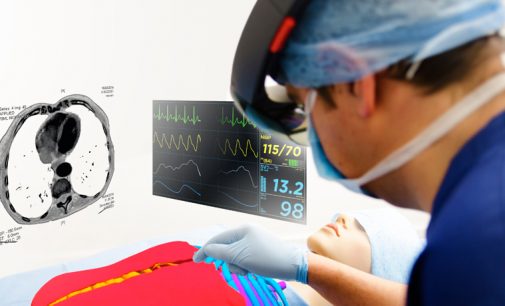
Product design and development company Cambridge Consultants is exploring how augmented reality (AR) could potentially be used in surgery to lead to better results for patients. By using Microsoft’s

Clinigen Group’s Idis Managed Access division and Tesaro have partnered to launch a Managed Access Program (also known as an Early Access Program) in Europe for niraparib, the investigational
Abbott (NYSE: ABT) today announced CE Mark and first use of the new Confirm RxTM Insertable Cardiac Monitor (ICM), the world’s first smartphone compatible ICM that will help physicians identify
A self-cleaning keyboard which claims to eliminate 99.99% of bacteria and pathogens has been developed by technology company Vioguard. The keyboard comes with its own case which acts as the
Proto Labs, digital manufacturer of prototypes and production parts, has expanded its 5-axis milling CNC machining capabilities across Europe. With the expansion of 5-axis CNC milling (also known as 3+2
Intuitive Surgical has bagged a CE mark for the latest product in its da Vinci series of robot-assisted surgical systems. The regulatory nod clears Intuitive Surgical to start selling a
Pharmaceutical wholesaler United Drug has announced a €40 million investment in technology and innovation at its headquarters at Citywest in Dublin. United Drug works with more than 1,800 pharmacies, hospitals,
On April 18, 2017 Fujifilm announced the completion of a $93-million cGMP production facility in Texas. The facility expansion is part of the company’s goal to increase the production capacity
Amryt Pharma, the orphan drug development company that was subject to a reverse takeover of former oil and gas group Fastnet last year, posted €1.35 million of sales last year.
Now that the die has been cast, it’s time for the deal-making process to begin – if indeed a deal is on the cards. It seems that every time a
Sharp Clinical Services, part of UDG Healthcare plc, has announced a £9 million ($11.2 million) investment to fund a new multiple-phase pharmaceutical manufacturing, packaging and distribution facility in Wales. The investment, which
The European Medicines Agency (EMA) wants to suspend around 300 marketed generic meds (and applications) coming out of Indian CRO
Drugmaker Mylan has recalled over 80,000 EpiPens across the world due to reports of the device failing to activate. The
Natoli Engineering Company, Inc. is expanding its European presence by launching its newest business division, Natoli International, and with that,
Healthcare giant Johnson & Johnson is planning to close its medical manufacturing plant in West Lothian, Scotland, potentially affecting around
Score one for Samsung Bioepis in its quest to bring a biosimilar version of AbbVie’s anti-inflammatory behemoth Humira to market in
Medical device company Olberon has developed a product to help doctors and nurses insert needles into patients’ blood. Olberon states
Dublin-headquartered pharma company Allergan is to invest €42 million in its Irish operations this year. The company, which employs approximately
In a landmark decision, the United States FDA and the European Commission announced on March 2, 2017 that they will recognize
The company’s latest report states that key drivers of the Russian healthcare market include improving regulatory guidelines and government initiatives
The Irish division of Crowthorne Hi-Tec Services (CHTS) has joined forces with Biopharma Group to offer combined services throughout the whole
The joint venture will combine Sanofi’s biologics development pipeline with Lonza’s expertise to design, construct, start-up and operate a state-of-the-art
Big Pharma companies Pfizer, Sanofi and Johnson & Johnson were among the big investors last year in the Flanders region of
Boston Scientific said on Thursday it was recalling a range of heart devices made at its Galway plant, sending the
On Feb. 23, 2017, Novasep announced the opening of its new €11-million (approximately $11.64 million) bioconjugation unit in the
Distek, Inc., a leading manufacturer of laboratory testing instruments for the pharmaceutical and biotechnology industry, as well as an experienced
The Austrian Centre of Industrial Biotechnology (acib) and GE Healthcare are introducing a cell line engineering research collaboration to bring
Jonathan Gardiner, packaging technologist at CDMO Almac looks at how packaging can become more efficient. The old adage, move with
Dublin-domiciled Medtronic, the world’s largest standalone medical device maker, saw profits more than double at its main Irish subsidiary last
Alnwick-based research and development company, Arc Trinova Ltd, trading as Arcinova, recently received approval for funding from the UK’s Rural
On Feb. 2, 2017, FDA issued a warning letter to Sato Pharmaceutical Co., Ltd. after inspectors found current good manufacturing
Roche’a anticipated approval of ocrelizumab will drive the company’s share of the market from <1% in 2016 to 15% by
Biopharmaceutical, analytical and bioanalytical contract solutions provider, SGS has announced that it is expanding its elemental analysis and testing capabilities
Global supply partner for drugs, biologics and consumer health products, Catalent, has announced that it has completed the acquisition of
Originally projected to be on the market by 2020, excitement about the contact lens to measure glucose levels from Novartis and Google
The company announced an investment in a new facility in Dundalk, County Louth, Ireland. In November 2016, the company