The European Medicines Agency (EMA) has published an action plan to improve the product information (PI) for EU medicines, an information package for patients and healthcare professionals that accompanies every single medicine authorised in

A £10 million Centre of Excellence in Precision Medicine has been launched by Invest Northern Ireland and Queen’s University Belfast. The Centre of Excellence in Precision Medicine will develop an

Diaceutics, the Irish data insights and solutions company helping patients receive potentially lifesaving medicine through better testing, won the Small & Emerging Exporter of the Year Award at the recent Export

Only by rethinking our health systems can we ensure that they remain fit-for-purpose and provide patient-centred care. This is what the 28 Country Health Profiles recently published by the Commission,

US biopharma company Regeneron Pharmaceuticals is to create 300 new jobs in Ireland as part of a new $100 million investment. The company has announced a further expansion of its

Public Health England has launched a major new campaign designed to further reign in inappropriate use of antibiotics, as estimates show that around 5,000 people in England alone die every

The FDA has approved Bydureon BCise for adults with type-2 diabetes whose blood sugar remains uncontrolled on one or more oral medicines. AstraZeneca has announced that the US Food and Drug Administration
Gilead’s Yescarta – recently acquired through its purchase of Kite Pharma -has become the second gene therapy to be approved by the US Food and Drug Administration, offering a new

AventaMed, a Cork-based medtech firm focused on simplifying surgical procedures, has secured a €1.8 million investment to enable the company to attain FDA approval and go to market in the

More than 70 Irish medical technology companies will seek investment from 300 international blue-chip company buyers from 42 countries on Thursday. Enterprise Ireland’s bi-annual Med in Ireland event at the RDS is

Double Helix Technologies (Doulix) announced it will collaborate with Agilent Technologies to promote Agilent’s SureVector next-generation cloning kits through Doulix’s web platform. Through this partnership, synthetic biologists will be able to

University of Liverpool researchers, working with F2G Limited (Eccles, Manchester), have developed a new antifungal drug to help in the treatment of life threatening invasive fungal infections such as invasive aspergillosis.

Tjoapack, a Dutch contract packaging organisation (CPO), has added an additional bottling line to its facility in Etten-Leur, the Netherlands, to meet growing demand for this service, particularly from generics

A retrospective cohort study, published in the British Medical Journal, has found no conclusive evidence that most oncology treatments approved for use by the European Medicines Agency (EMA) offered survival

A 3D printed part can combine 20 parts into a single part and improve performance, reducing parts in a manufacturing process could benefit the biomanufacturing industry where processes and equipment
The Cell and Gene Therapy Catapult (CGT Catapult) has signed a Memorandum of Understanding (MoU) with the Forum for Innovative Regenerative Medicine (FIRM) in Japan, demonstrating a shared ambition to

Pharmaceutical outsourcing provider, PCI Pharma Services, has acquired Dublin-based pharmaceutical and healthcare contract packaging services provider, Millmount Healthcare. “We are very excited to welcome the Millmount team to PCI,” commented
A strategic alliance between Sygnature Discovery, provider of integrated drug discovery resource and expertise, and ApconiX, non-clinical safety strategies and ion channel screening experts, has been announced. The partnership will
As the new academic year begins, the Medicines & Healthcare products Regulatory Agency (MHRA) is campaigning to students about the risks of self-prescribing and self-medicating with medicines purchased online. Buying
Losing valuable staff is the major concern in the impending relocation of the European Medicines Agency. The agency which is
The Food and Drug Administration is requiring manufacturers of the most widely prescribed painkillers to provide extensive training to doctors

The Brexit-inspired need to move the London HQ of the European Medicines Agency will throw up two major risks when
The antibody drug conjugate (ADC) development company, Glythera, has been granted exclusive, worldwide rights to Cancer Research UK’s novel CDK11

Australian-based medical grade cannabis company, MGC Pharmaceuticals, will sponsor and speak at the first conference on medical cannabis in the

Janssen has announced that the European Commission (EC) has approved its darunavir-based single-tablet regimen (STR), Symtuza▼, for the treatment of

Bio/pharmaceutical analytical and bioanalytical contract solutions provider, SGS, has entered into a partnership with Nature’s Fingerprint, which specialises in product

If you’ve ever wanted to contribute to a science event that could potentially help save lives then a new app
UCB has announced that the European Commission (EC) has approved expanding the use of its anti-epileptic drug (AED) Vimpat (lacosamide)

Doctors and not pharmacies should retain control of any decision to switch a patient from a modern biologic medicine to

Kymab, an emerging biopharmaceutical company focused on the discovery and development of fully human monoclonal antibody drugs, announced new data
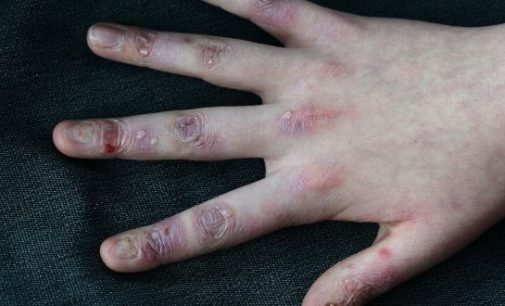
Irish woundcare group Amryt has become the front-runner in the race to find a treatment for epidermolysis bullosa (EB), known

ReSolve — ‘Renewable solvents with high performance in applications and improved toxicity profiles’ — is set to last three years

The Food and Drug Administration (FDA) has approved BioMarin Pharmaceutical’s bulk biologics manufacturing plant, located in Shanbally, Cork, Ireland for

British manufacturers are asking the government to negotiate access to the single market and a form of customs union along
The UK National Institute for Health and Care Excellence (NICE) has granted approval for Roche’s breast cancer drug Kadcyla for

BioPharmaChem Ireland, the Ibec group that represents business in the sector, said the announcement of MSD to invest €280 million

Novo Nordisk has won EU approval for its new haemophilia drug Reflixia and is planning its first European launches in
The European Medicines Agency’s Pharmacovigilance Risk Assessment Committee (PRAC) discussed the safety reviews of a variety of medications during its

The European Commission (EC) has approved Johnson & Johnson’s proposed $30bn acquisition of Actelion Pharmaceuticals, subject to conditions J&J signed

Medtech firm Health Beacon, whose backers include Bill McCabe’s Oyster Capital, will more than double its Dublin workforce with the

The European Medicines Agency (EMA) and the European Commission have published an information guide for healthcare professionals on biosimilar medicines. Biosimilars are

The NHS could be faced with having to pay nearly half a billion pounds more if British expats decide to

Three new apprenticeship schemes have been introduced in the medtech sector as part of Government attempts to plug the skills
Cambrex, manufacturer of small molecule active pharmaceutical ingredients (APIs), has announced it is investing in new capacity and continuous flow
The European Commission (EC) has granted marketing authorisation for AstraZeneca’s Brilique (ticagrelor) orodispersible tablets (ODT) as a new method of

A genetic variant that protects the heart against cardiovascular disease has been discovered by researchers at the Wellcome Trust Sanger

The UK has the strongest and most robust clinical pipeline in Europe, with the number of drug products in development
The European Medicines Agency (EMA) met with a delegation from the East African Community (EAC) from May 18–19, 2017 as

The Regulatory Affairs Professionals Society (RAPS) is hosting a dedicated workshop on the new EU regulations for medical devices and

Novartis has revealed that up to 500 positions in Switzerland will be eliminated or transferred to other areas over the











