Public health authorities and vaccine makers have debated whether an omicron-specific booster or additional shots will be necessary to protect those already immunized as the COVID-19 pandemic continues. While data
Medical Research

Intriguing antitumor activity is found in a very promising class of natural compounds: cyclic depsipeptides, which have a challenging structure that makes their investigation difficult. Now, Chinese scientists have established

US biopharma company Regeneron Pharmaceuticals is to create 300 new jobs in Ireland as part of a new $100 million investment. The company has announced a further expansion of its

CN Bio Innovations Limited has announced today that it has entered into a Research Collaboration Agreement with the US Food and Drug Administration’s (FDA) Center for Drug Evaluation and Research
EGFR is a common genetic target in lung cancer, but not all EGFR mutations are created equal. Patients with a type of EGFR anomaly called an “EGFR exon 20 insertion”
Ketamine, a medication commonly used for pain relief and increasingly used for depression, may help alleviate migraine pain in patients who have not been helped by other treatments. The study

API Warning Letters Each spring, the FDA publishes data on its website related to Form 483 inspection observations and warning letters issued by the Centre for Drug Evaluation and Research (CDER) during

Public Health England has launched a major new campaign designed to further reign in inappropriate use of antibiotics, as estimates show that around 5,000 people in England alone die every

The FDA has approved Bydureon BCise for adults with type-2 diabetes whose blood sugar remains uncontrolled on one or more oral medicines. AstraZeneca has announced that the US Food and Drug Administration
In a decades-long game of hide and seek, scientists from Sydney’s Westmead Institute for Medical Research have confirmed for the very first time the specific immune memory T-cells where infectious
Eli Lilly has signed up CureVac to develop cancer vaccines based on its mRNA technology, in a deal that could potentially be worth more than $2.6 billion to the German
Gilead’s Yescarta – recently acquired through its purchase of Kite Pharma -has become the second gene therapy to be approved by the US Food and Drug Administration, offering a new

More than 70 Irish medical technology companies will seek investment from 300 international blue-chip company buyers from 42 countries on Thursday. Enterprise Ireland’s bi-annual Med in Ireland event at the RDS is
Plasticell, a biotechnology company specialising in stem cell screening and cell therapy development, has entered into a collaboration with GSK to use its combinatorial stem cell screening technology, CombiCult, to
A specialist in therapeutic approaches to treat resistant bacterial infections, Mutabilis has been selected to join the European consortium ENABLE — which is aimed at developing antibiotics to tackle infections

The FDA has approved an expanded indication for Stelara for the treatment of adolescents with moderate plaque psoriasis. Janssen Biotech, Inc., has announced that the U.S. Food and Drug Administration
SGS announced it has entered into collaboration with Bavarian Nordic A/S, an international biotechnology company specialising in vaccines, to develop a new respiratory syncytial virus (RSV) challenge strain. This will

Myeloma UK has launched a nationwide trial aiming to assess the effectiveness of a novel treatment combination that includes Darzalex for patients with genetically-defined high-risk myeloma. The trial, which will
A team of researchers from Imperial College London have used machine learning to develop a device that could potentially improve

IBS scientists at the Center for Self-Assembly and Complexity, within the Institute for Basic Science (IBS), invented a hydrogel to

The first open-access biotech laboratory has opened in Manchester, affording early-stage biotech and medtech companies, as well as entrepreneurial individual

Ahead of World Mental Health Day, a new project that uses connected technologies has been developed by the NHS to

A retrospective cohort study, published in the British Medical Journal, has found no conclusive evidence that most oncology treatments approved
Scientists have had positive results from a laboratory-based study using a well-known alcohol aversion drug disulfiram, to combat chemotherapy resistance
The BBC has reported that researchers from the University of Oxford’s Jenner Institute are seeking 500 NHS patients to test

The cancer immunotherapy pipeline, displays a high level of first-in-class innovation with novel developments focusing heavily on producing the next
Scientists at the University of Washington (UW) have designed mini-protein binders on a computer platform, which are not naturally occurring

A 3D printed part can combine 20 parts into a single part and improve performance, reducing parts in a manufacturing
The Cell and Gene Therapy Catapult (CGT Catapult) has signed a Memorandum of Understanding (MoU) with the Forum for Innovative

The quality of medicines information resources needs to be improved, according to a new statement of policy from the International
The U.S. Food and Drug Administration is opening a new front in its efforts to reduce high drug prices by
As the new academic year begins, the Medicines & Healthcare products Regulatory Agency (MHRA) is campaigning to students about the
The antibody drug conjugate (ADC) development company, Glythera, has been granted exclusive, worldwide rights to Cancer Research UK’s novel CDK11

Australian-based medical grade cannabis company, MGC Pharmaceuticals, will sponsor and speak at the first conference on medical cannabis in the

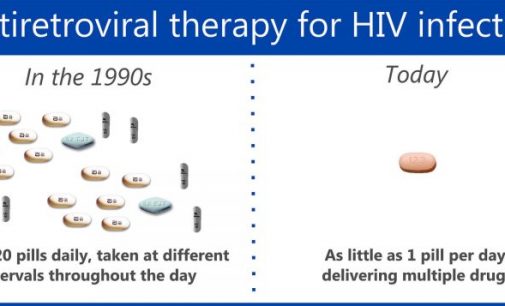
Janssen has announced that the European Commission (EC) has approved its darunavir-based single-tablet regimen (STR), Symtuza▼, for the treatment of

Kymab, an emerging biopharmaceutical company focused on the discovery and development of fully human monoclonal antibody drugs, announced new data
The European Medicines Agency’s Pharmacovigilance Risk Assessment Committee (PRAC) discussed the safety reviews of a variety of medications during its
Pfizer has secured approval the European Commission (EC) for TRUMENBA (Meningococcal Group B Vaccine) for preventing invasive meningococcal disease caused

Researchers at Karolinska Institutet in Sweden have managed to synthesise lung surfactant, a drug used in the care of preterm
The European Medicines Agency (EMA) has approved Zebinix (eslicarbazepine acetate) for use as once-daily monotherapy to treat adults with newly-diagnosed

World Cancer Research Fund International has released a new report that demonstrates an increased risk of breast cancer is associated

Veltassa (patiromer), developed by Relypsa, has received a recommendation for marketing approval from the Committee for Medicinal Products for Human

A new study has shown that an ingestible gastric balloon could be used to help obese people lose weight without
From diagnosis of HIV to successful viral suppression: this new ECDC report summarises key findings concerning and the Continuum of
An ambitious collaboration between Brainomix, an emerging British business, and Boehringer Ingelheim, one of the world’s largest drug companies, is
Pfizer has announced it will provide its breast cancer drug, palbociclib — which has been provisionally rejected by NICE for
A clinical trial funded by Arthritis Research UK and the National Institute for Health Research (NIHR) led by professors from
A late stage clinical study of Aeterna Zentaris’ Zoptrex in women with locally advanced, recurrent or metastatic endometrial cancer has