A £10 million Centre of Excellence in Precision Medicine has been launched by Invest Northern Ireland and Queen’s University Belfast. The Centre of Excellence in Precision Medicine will develop an
Laboratory
The European Medicines Agency’s Pharmacovigilance Risk Assessment Committee (PRAC) discussed the safety reviews of a variety of medications during its June 2017 meeting. The medicines in review included treatments for

A new study has shown that an ingestible gastric balloon could be used to help obese people lose weight without the need for invasive surgery. When swallowed the Elipse balloon
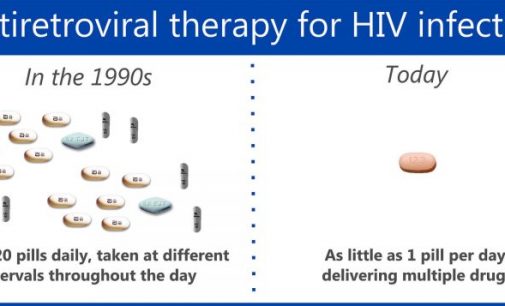
From diagnosis of HIV to successful viral suppression: this new ECDC report summarises key findings concerning and the Continuum of HIV Care in Europe based on data reported by countries

Preclinical results of research by City College of New York scientists and TechnoVax, Inc. in animal models demonstrate favorable outcomes in developing a vaccine against the mosquito-borne Zika virus.
Poxel was able to score positive results for its type 2 diabetes drug in a Phase IIb study in Japan, preparing the company to go for a Phase III program. Poxel, one of
Fungi are a potential goldmine for the production of pharmaceuticals. This is shown by researchers at Chalmers University of Technology, who have developed a method for finding new antibiotics from
The first patient has been recruited in an exploratory Phase Ib clinical trial of Targovax’s second product from its RAS-peptide immunotherapy platform, TG02. In the open-label, non-randomised trial — to
Allergan, a global biopharmaceutical company, announced that it has entered into a clinical trial agreement with Novartis to conduct a Phase 2b study, using Allergan’s cenicriviroc (CVC) and Novartis’ lead FXR
Two startups in the fields of synthetic biology, Biosyntia and FGen, have struck a partnership to identify promising microbial strains. The objective is to find a strain that enables bioproduction
Allergan plc, a global pharmaceutical company announced topline data from a phase II study with major depressive disorder (MDD). The study evaluated the efficacy, safety and tolerability of a single administration
The European Commission has approved Novartis’ Tafinlar (dabrafenib) in combination with Mekinist (trametinib) for the treatment of patients with BRAF V600-positive advanced or metastatic non-small cell lung cancer (NSCLC). The
Scientists at EPFL and NTU have discovered that combining an anticancer drug with an antirheumatic produces improved effects against tumors. The discovery opens a new path for drug-drug synergy. One
The agency is recommending the suspension of a variety of medications because of unreliable bioequivalence studies conducted by Micro Therapeutic Research Labs The European Medicines Agency (EMA) announced on March
PureTech will take on the development of two mTORC1 inhibitors from Novartis aimed at stopping the decline of the immune system associated with age. Novartis is building up the portfolio
A new study published in PLOS Medicine’s Special Issue on Dementia has found that the metabolism of omega-3 and omega-6 unsaturated fatty acids in the brain are associated with
Researchers at Trinity College Dublin have discovered a way to prevent bacteria from growing on medical devices that are implanted in the human body. The researchers state the discovery is
Newron, an Italian biotech focused on CNS diseases, is on a roll. The latest win? FDA approval of its Parkinson’s drug, Xadago. Just in time for World Parkinson’s Day on
Shire has secured label expansion approval from the European Commission for its hereditary angioedema therapy Cinryze. The European Commission (EC) has approved the label extension application, granting three new indications
Cerenis therapeutic’s lead candidate CER-001 has failed Phase II for the treatment of patients with post-acute coronary syndrome (ACS). The French-based Biotech Cerenis is developing therapies for the treatment ofcardiovascular and
Novartis has received FDA approval for Kisqali, a first-line treatment for breast cancer originally developed by the British biotech Astex Pharmaceuticals. The oncology company Astex Pharmaceuticals, located in Cambridge’s biotech hub, entered
Scientists from the University of Cambridge have determined the first 3D structures of mammalian genomes from individual cells. For the
MedImmune, the global biologics research and development arm of AstraZeneca, and Sanofi Pasteur, the vaccines division of Sanofi, have announced
Abeona Therapeutics Inc., a clinical-stage biopharmaceutical company focused on developing gene therapies for life-threatening rare diseases, announced today that the
Italy adopts gender-neutral HPV vaccination program, citing study by health economics expert from Kingston University London’s Business School. The Italian
Medical device company Olberon has developed a product to help doctors and nurses insert needles into patients’ blood. Olberon states
Regulators on both sides of the Atlantic are reviewing three new submissions for medicines containing ertugliflozin, an investigational SGLT2 inhibitor
Actelion has received approval from the European Commission for Ledaga, a treatment to prevent the progression of a rare form
A new method to help diagnose oesophageal cancer is being researched by engineers and physicians at the Institute of Biological
Diagnostics company, Owlstone Medical, who is developing a breathalyser for disease has announced it has signed a master service agreement
Researchers at the Universities of Dundee and Edinburgh in Scotland are looking to work with the pharmaceutical industry to improve
Sanofi and its vaccines global business unit Sanofi Pasteur announced today an agreement with MedImmune, the global biologics research and development arm of
A French teen who was given gene therapy for sickle cell disease more than two years ago now has enough
On 1 March the newly established European Reference Networks (ERNs) has started their work. ERNs are unique and innovative cross-border
29 – 31 March 2017, Glasgow, United Kingdom. DIA, (founded as the Drug Information Association) announced critical workshops to be
The World Health Organization has issued a list of the top dozen bacteria most dangerous to humans, warning that doctors
The Irish division of Crowthorne Hi-Tec Services (CHTS) has joined forces with Biopharma Group to offer combined services throughout the whole
The joint venture will combine Sanofi’s biologics development pipeline with Lonza’s expertise to design, construct, start-up and operate a state-of-the-art
FDA’s Office of Generic Drugs (OGD) released their 2016 Annual Report on Feb. 24, 2017. The report details the office’s accomplishments
The European Medicines Agency (EMA) announced on Feb. 24, 2017 that its Committee for Medicinal Products for Human Use (CHMP) has
GlaxoSmithKline plc and Innoviva, Inc. announced positive headline results from a non-inferiority lung function study, which demonstrated that patients with well-controlled asthma were
The University of Surrey has launched a new academic programme, aimed to help change the delivery of healthcare in the
The Austrian Centre of Industrial Biotechnology (acib) and GE Healthcare are introducing a cell line engineering research collaboration to bring
Roche’s oral ALK inhibitor, Alecensa, has been conditionally approved in the European Union as a monotherapy for anaplastic lymphoma kinase-positive
Lanthio Pharma has initiated a Phase I clinical trial with its lanthipeptide candidate MOR107, which is being developed as a
Scientists at Emory University’s Winship Cancer Institute are on a mission to unlock the mysteries of lung cancer cells that
Researchers at Eindhoven University of Technology (TU/e) present a new method that should enable controlled drug delivery into the bloodstream
Cambridge Research Biochemicals (CRB), specialist in the synthesis of custom peptides and antibody production has announced the release of its new
Faron Pharmaceuticals has recruited its first patient to a phase II clinical trial of its lead drug, Traumakine, in the
UK-based rare and speciality diseases group Mereo BioPharma says its brittle bone disease drug BPS-804 has been accepted to participate
On Feb. 2, 2017, FDA issued a warning letter to Sato Pharmaceutical Co., Ltd. after inspectors found current good manufacturing
Modus Therapeutics has raised €3.4M to support the completion of a Phase II trial for sickle cell disease, a painful condition