Canada is set to be the first country to give citizens the option for a vegetarian coronavirus shot. The world’s first plant-derived Covid vaccine, Covifenz, was cleared for use in
Approval

The Food and Drug Administration (FDA) has approved BioMarin Pharmaceutical’s bulk biologics manufacturing plant, located in Shanbally, Cork, Ireland for production of the formulated bulk substance (N-acetylgalactosamine 6-sulfatase (GALNS) used
The UK National Institute for Health and Care Excellence (NICE) has granted approval for Roche’s breast cancer drug Kadcyla for routine use within the NHS region. NICE has recommended Kadcyla

Novo Nordisk has won EU approval for its new haemophilia drug Reflixia and is planning its first European launches in the fourth quarter. The European Commission marketing authorisation comes one

The European Commission (EC) has approved Johnson & Johnson’s proposed $30bn acquisition of Actelion Pharmaceuticals, subject to conditions J&J signed an agreement in January this year to acquire Actelion, which develops
Pfizer has secured approval the European Commission (EC) for TRUMENBA (Meningococcal Group B Vaccine) for preventing invasive meningococcal disease caused by Neisseria meningitidis serogroup B (MenB) in individuals 10 years

The European Medicines Agency (EMA) has approved Aptar Pharma’s integrated electronic nasal lockout device (e-Lockout) following a multi-year development with Takeda Pharmaceuticals International. Aptar Pharma agreed to supply Takeda with its
The European Commission (EC) has granted marketing authorisation for AstraZeneca’s Brilique (ticagrelor) orodispersible tablets (ODT) as a new method of treatment administration. This decision means that ticagrelor will become the
The European Medicines Agency (EMA) has approved Zebinix (eslicarbazepine acetate) for use as once-daily monotherapy to treat adults with newly-diagnosed partial-onset epilepsy. It is already indicated in Europe as an

The EC granted full marketing approval for Biogen’s Fampyra® (prolonged-release fampridine) as a treatment to improve walking in people with multiple sclerosis (MS). Clearance in Europe was based on the

Hansa Medical has accessed the EMA’s priority medicines scheme to accelerate the development of a therapy that broadens the access to kidney transplants. Hansa Medical, based in Lund, Sweden, develops

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval to expand the use of Novartis’ Zykadia (ceritinib) in anaplastic lymphoma kinase (ALK)-positive non-small cell

Veltassa (patiromer), developed by Relypsa, has received a recommendation for marketing approval from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) for the
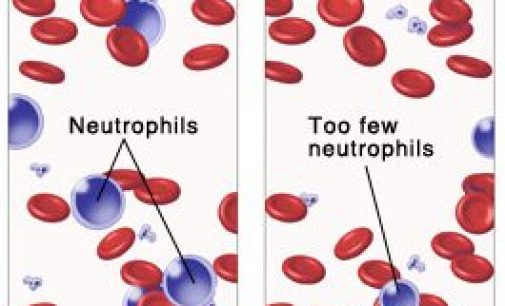
Cinfa Biotech announced trial results for a biosimilar of Amgen’s Neulasta, a multi-billion blockbuster to treat chemotherapy-induced neutropenia. Cinfa Biotech is a Spanish company specialized in developing biosimilars. The biotech
Abbott (NYSE: ABT) today announced CE Mark and first use of the new Confirm RxTM Insertable Cardiac Monitor (ICM), the world’s first smartphone compatible ICM that will help physicians identify
Intuitive Surgical has bagged a CE mark for the latest product in its da Vinci series of robot-assisted surgical systems. The regulatory nod clears Intuitive Surgical to start selling a
Impax Laboratories, Inc., a specialty pharmaceutical company, announced it has received final U.S. Food and Drug Administration (FDA) approval for a generic version of Vytorin (ezetimibe/simvastatin tablets), 10/10, 10/20, 10/40 and 10/80
Contrary to some political claims, the U.S. Food and Drug Administration approved more drugs, and two to three months faster on average, than European regulators did in recent years, new research shows. “It’s
The European Commission has approved Novartis’ Tafinlar (dabrafenib) in combination with Mekinist (trametinib) for the treatment of patients with BRAF V600-positive advanced or metastatic non-small cell lung cancer (NSCLC). The
Xeljanz (tofacitinib citrate) receives marketing authorization in the European Union for the treatment of moderate to severe active rheumatoid arthritis (RA). Pfizer Inc. announced today that the European Commission (EC)
Apeiron Biologics has announced that the EMA’s CHMP has recommended the approval of APN311 for immunotherapy of high risk neuroblastoma. Apeiron Biologics is a Vienna-based biotech company developing immunological approaches
Newron, an Italian biotech focused on CNS diseases, is on a roll. The latest win? FDA approval of its Parkinson’s drug, Xadago. Just in time for World Parkinson’s Day on
Debiopharm International, a Swiss-based company, part of Debiopharm Group, announced that triptorelin 6-month formulation (Decapeptyl and Pamorelin 22.5 mg) received
Novartis has received FDA approval for Kisqali, a first-line treatment for breast cancer originally developed by the British biotech Astex






