The quality of medicines information resources needs to be improved, according to a new statement of policy from the International
- Brexit – Implications For the Pharma Industry in Ireland The UK’s referendum decision to leave the EU in May 2016 undoubtedly sent shockwaves throughout Europe. European countries are still struggling to interpret what ‘Brexit’ will mean for them. Ireland,...
- EMA to Work With Stakeholders to Improve Product Information For EU Medicines The European Medicines Agency (EMA) has published an action plan to improve the product information (PI) for EU medicines, an information package for patients and healthcare professionals that accompanies every single medicine authorised in...
- Diaceutics Wins at Export Industry Awards Diaceutics, the Irish data insights and solutions company helping patients receive potentially lifesaving medicine through better testing, won the Small & Emerging Exporter of the Year Award at the recent Export...
- Brexit – Key Issues For the Pharmaceutical Industry Brexit – the stark reality is that the UK’s EU membership and the application of all EU legislation in the UK ends automatically at midnight on 30th March 2019! Current understanding...
- European Commission Diagnoses the State of Health in the EU Only by rethinking our health systems can we ensure that they remain fit-for-purpose and provide patient-centred care. This is what the 28 Country Health Profiles recently published by the Commission,...

The U.S. Food and Drug Administration is opening a new front in its efforts to reduce high drug prices by
A strategic alliance between Sygnature Discovery, provider of integrated drug discovery resource and expertise, and ApconiX, non-clinical safety strategies and
As the new academic year begins, the Medicines & Healthcare products Regulatory Agency (MHRA) is campaigning to students about the
Losing valuable staff is the major concern in the impending relocation of the European Medicines Agency. The agency which is
The Food and Drug Administration is requiring manufacturers of the most widely prescribed painkillers to provide extensive training to doctors

The Brexit-inspired need to move the London HQ of the European Medicines Agency will throw up two major risks when
The antibody drug conjugate (ADC) development company, Glythera, has been granted exclusive, worldwide rights to Cancer Research UK’s novel CDK11

Australian-based medical grade cannabis company, MGC Pharmaceuticals, will sponsor and speak at the first conference on medical cannabis in the

Janssen has announced that the European Commission (EC) has approved its darunavir-based single-tablet regimen (STR), Symtuza▼, for the treatment of

Bio/pharmaceutical analytical and bioanalytical contract solutions provider, SGS, has entered into a partnership with Nature’s Fingerprint, which specialises in product

Camino Pharma, LLC, a new start-up company focusing on finding cures for cancer and brain disorders, announced that its Co-founder

If you’ve ever wanted to contribute to a science event that could potentially help save lives then a new app
UCB has announced that the European Commission (EC) has approved expanding the use of its anti-epileptic drug (AED) Vimpat (lacosamide)

The potential for harmful chemicals to be extracted from packaging materials that pose a risk to patients has spurred regulatory

Doctors and not pharmacies should retain control of any decision to switch a patient from a modern biologic medicine to

Kymab, an emerging biopharmaceutical company focused on the discovery and development of fully human monoclonal antibody drugs, announced new data
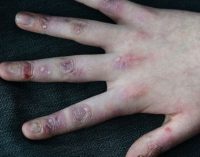
Irish woundcare group Amryt has become the front-runner in the race to find a treatment for epidermolysis bullosa (EB), known














