FDA Gives Positive Review Of Aerie’s Experimental Glaucoma Drug
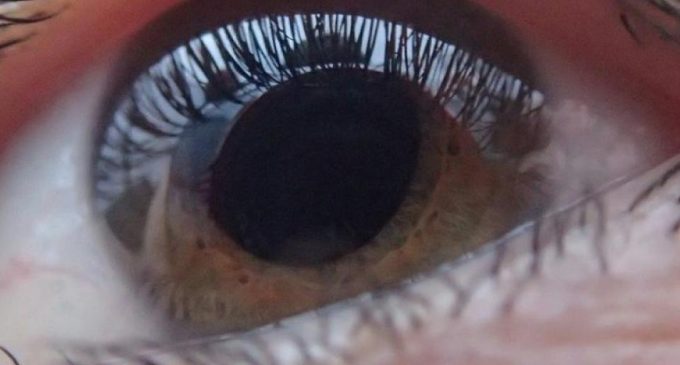
A study by the U.S. Food and Drug Administration has backed up Aerie Pharmaceutical’s evidence that its experimental drug is effective in treating glaucoma.
According to Reuters, the results were from a preliminary review, and the agency is still waiting on advice from outside experts. But upon news of the findings, Aerie’s shares jumped by 19 percent.
Glaucoma is often caused by a buildup of pressure inside the eye, which can damage the optic nerve and eventually cause permanent vision loss. In fact, it is the second leading cause of blindness around the world.
The number of Americans with glaucoma is expected to rise in the coming years. By 2030, an estimated 4 million Americans will have glaucoma — up from 2.7 million who have it now.
The drugs currently on the market that typically treat glaucoma are prostaglandins such as Pfizer’s Xalatan and Novartis AG’s Travatan. This class of drugs help lower eye pressure.
Aerie’s drug, called Rhopressa, is also designed to lower pressure but instead targets the main drain for eye fluid, called the trabecular network. The treatment is also supposed to have a lower risk of pigmentation and changes to eyelash length.
Rhopressa is a once-daily treatment; and the FDA also concluded that a double dose did not help those with a higher buildup of pressure.






