A 3D printed part can combine 20 parts into a single part and improve performance, reducing parts in a manufacturing process could benefit the biomanufacturing industry where processes and equipment
Posts From editor
The Cell and Gene Therapy Catapult (CGT Catapult) has signed a Memorandum of Understanding (MoU) with the Forum for Innovative Regenerative Medicine (FIRM) in Japan, demonstrating a shared ambition to

Pharmaceutical outsourcing provider, PCI Pharma Services, has acquired Dublin-based pharmaceutical and healthcare contract packaging services provider, Millmount Healthcare. “We are very excited to welcome the Millmount team to PCI,” commented

The quality of medicines information resources needs to be improved, according to a new statement of policy from the International Pharmaceutical Federation (FIP). This statement, ‘strategic development of medicines information
The U.S. Food and Drug Administration is opening a new front in its efforts to reduce high drug prices by encouraging development of generic versions of hard-to-make medicines. Complex drugs
A strategic alliance between Sygnature Discovery, provider of integrated drug discovery resource and expertise, and ApconiX, non-clinical safety strategies and ion channel screening experts, has been announced. The partnership will
As the new academic year begins, the Medicines & Healthcare products Regulatory Agency (MHRA) is campaigning to students about the risks of self-prescribing and self-medicating with medicines purchased online. Buying
Losing valuable staff is the major concern in the impending relocation of the European Medicines Agency. The agency which is responsible for the evaluation, authorisation and pharmacovigilance of all medicines
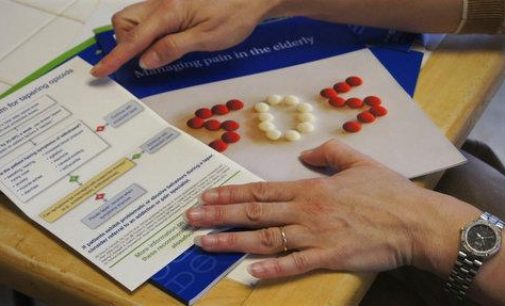
The Food and Drug Administration is requiring manufacturers of the most widely prescribed painkillers to provide extensive training to doctors in an attempt to reduce the number of patients who

The Brexit-inspired need to move the London HQ of the European Medicines Agency will throw up two major risks when it comes to patients and the approval of new medicines,
The antibody drug conjugate (ADC) development company, Glythera, has been granted exclusive, worldwide rights to Cancer Research UK’s novel CDK11 inhibitor programme for the development of multiple ADCs. Under the

Australian-based medical grade cannabis company, MGC Pharmaceuticals, will sponsor and speak at the first conference on medical cannabis in the UK — CannaTech UK Innovation Summit. “We are extremely happy

Janssen has announced that the European Commission (EC) has approved its darunavir-based single-tablet regimen (STR), Symtuza▼, for the treatment of HIV-1 in adults and adolescents in Europe. “Today’s decision by

Bio/pharmaceutical analytical and bioanalytical contract solutions provider, SGS, has entered into a partnership with Nature’s Fingerprint, which specialises in product and process authentication using natural tracer, to combat pharmaceutical counterfeiting.

Camino Pharma, LLC, a new start-up company focusing on finding cures for cancer and brain disorders, announced that its Co-founder and Head of Drug Discovery, Nicholas Cosford, Ph.D., has been awarded

If you’ve ever wanted to contribute to a science event that could potentially help save lives then a new app will let you do just that. The BBC Pandemic app
UCB has announced that the European Commission (EC) has approved expanding the use of its anti-epileptic drug (AED) Vimpat (lacosamide) as monotherapy and adjunctive therapy in the treatment of partial-onset

The potential for harmful chemicals to be extracted from packaging materials that pose a risk to patients has spurred regulatory authorities to establish guidelines for extractable and leachable (E&L) testing

Doctors and not pharmacies should retain control of any decision to switch a patient from a modern biologic medicine to a biosimilar version, the industry has argued. In its submission

Kymab, an emerging biopharmaceutical company focused on the discovery and development of fully human monoclonal antibody drugs, announced new data published in Science Translational Medicine indicate that Kymab’s most advanced
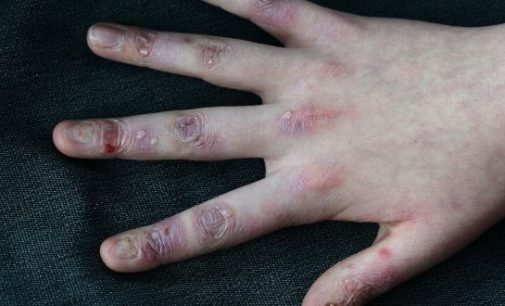
Irish woundcare group Amryt has become the front-runner in the race to find a treatment for epidermolysis bullosa (EB), known as butterfly disease which causes skin in mainly young patients
The materials science institute AMBER, headquartered at Trinity College in Dublin, will continue its cooperation with Nokia Bell Labs and
As recently published in the International Journal of Cancer, researchers from Breast-Predict, may have found a new drug for the








